Abstract

Introduction: Allogeneic stem cell transplantation (HSCT) from either matched siblings (MSD) or matched unrelated donors (MUD) remains a standard option for children with high risk acute lymphoblastic leukemia (ALL), either in first complete remission (CR) or after relapse. The superiority of total body irradiation (TBI)/VP16 conditioning, compared to a chemotherapy based conditioning (fludarabine and thiotepa plus either busulfan or treosulfan) was demonstrated in theprospective, international, randomized non inferiority phase III study which enrolled 417 patients beyond the age of 4 years (y) transplanted for ALL in CR from either MSD or MD (Peters et al, JCO 2021; FORUM study).
Besides the 201 patients randomized and actually receiving TBI from 2013 and 2018 in the randomizing countries, the FORUM trial included 411 additional children conditioned with TBI/VP16, enrolled either in countries where the randomization could not take place (for either legal or technical reasons) or in randomizing countries where TBI was chosen because the randomization was either skipped by physicians or families or had been discontinued.
Methods: Here we report the 3-year consolidated results of these 612 patients ³4y and <21y treated in >100 pediatric centers in 26 countries following HSCT from either MSD (n=186, 30%) or MUD (defined as 9 or 10/10 4-digit molecular HLA-compatibility, n=426, 70%) after 12Gy TBI-VP16 for ALL in CR³1. Transplant details and statistical methods werepreviously reported in detail (Peters et al, JCO 2021). Of note, the main difference between the two groups was the GvHD prophylaxis consisting of cyclosporine A (CSA) +/- methotrexate (MTX) in MSD and serotherapy + CSA + MTX (Day 1, 3, 6, 11 post-HSCT) in MUD.
Results: Patient's characteristics are reported in Table. Median age was 10y (4.0-20.8). The MSD and MUD groups were comparable, with the only exception of a larger use of peripheral blood and cord blood as stem cell source in the MUD population. In total, 299 pts were transplanted in CR1 (MSD, n=84; MUD, n=215) and 312 (MSD, n=102; MUD, n=210) in > CR1.
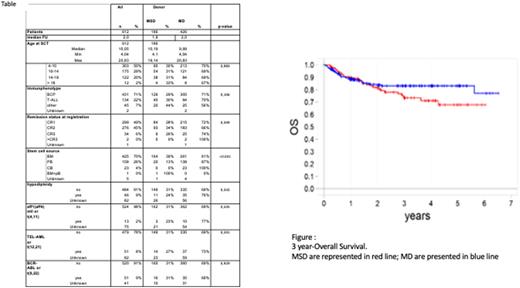
In univariate analysis (figure), the Kaplan-Meier estimate of 3-year overall survival (OS) was significantly better in the MUD compared to the MSD group (83±2% vs 73±5%, respectively, P=0.041). Both the 3-year cumulative incidence (CI) of treatment-related mortality (TRM) and the 3-year probability of Event Free Survival (EFS) were comparable in the 2 groups analyzed (7±3% vs 7±1%, P=0.87 and 69±5% vs 83±2%, P=0.25, in MSD and MUD, respectively). The 3-year cumulative incidence of relapse (CIR) was higher in MSD (24±4% and 16±2%, respectively), although not statistically significant (p=0.08). The CI of acute GvHD was comparable in both groups, whereas the 3-year CI of cGvHD was higher in MSD than in MUD (19±3 vs 11±2%, P=0.07). The GvHD-relapse-secondary malignancy-free survival (GRFS) is significantly better in MUD than MSD (62±3 vs ±51±5%, P=0.04).
In multivariate analysis, factors associated with superior OS include being transplanted from unrelated donor MUD, in CR1 and below the age of 14 years. Disease status at time of HSCT (i.e. CR1) was the only factor associated with better EFS and lower risk of post-transplant relapse.
Conclusion: Our data indicate that the use of MUD is no longer a risk factor in HSCT for childhood ALL. The greater immune-genetic disparity in the matched unrelated donor/recipient pairs protects from the risk of disease recurrence, while the use of serotherapy prevents the occurrence of chronic GvHD, with both factors contributing to the observed improved GRFS in patients transplanted from an unrelated donor.
Disclosures
Dalle:Novartis: Honoraria; Sanofi: Honoraria; Jazz Pharmaceuticals: Honoraria; Medac: Honoraria; Vertex: Honoraria; Orchard: Honoraria; Teva: Current equity holder in private company. Bader:Jazz: Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Research Funding; Neovii: Research Funding; Riemser: Research Funding, Speakers Bureau; Medac: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Miltenyi: Speakers Bureau. Locatelli:Medac: Speakers Bureau; Jazz Pharmaceuticals: Honoraria; Amgen: Speakers Bureau; Neovii: Speakers Bureau; BlueBird bio: Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Miltenyi: Speakers Bureau; SOBI: Speakers Bureau. Ifversen:Novartis: Membership on an entity's Board of Directors or advisory committees. Buechner:Jannsen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Díaz-de-Heredia:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; Biotest: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses . Peters:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Medac: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Riemser: Consultancy, Speakers Bureau; Jazz: Other, Research Funding; Neovii: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal